CAS 119752-83-9, Cat. No EN300-321062
Reagent SO2 source
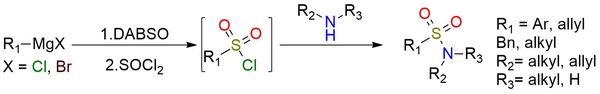
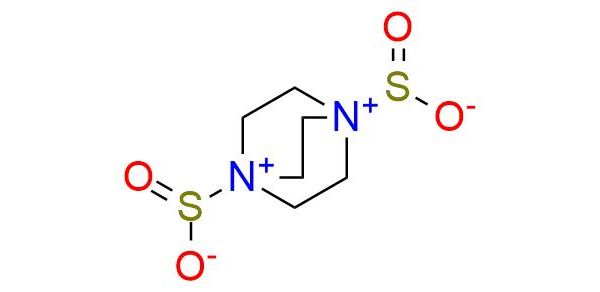
DABSO is a bench-stable colorless solid suitable for use in organic synthesis as a replacement for gaseous sulfur dioxide1. The complex can be combined with Grignard reagents to form sulfinates, which can then be converted in situ to a series of sulfonamides1,2. Alternatively, a reaction with anilines and iodine leads to the formation of a series of sulfamides. Cheletropic addition between DABSO and 2,3-dimethylbutadiene provides the corresponding sulfolene2. This versatile compound finds application as an efficient reagent, offering enhanced convenience and control in the synthesis of sulfinates, sulfonamides, and related derivatives.
Synonyms: 1,4-diazabicyclo[2.2.2]octane sulfur dioxide complex; 1,4-diazabicyclo[2.2.2]octane-1,4-diium-1,4-disulfinate; 1,4-diazobicyclo[2.2.2]octane-bis(sulfur dioxide) adduct; 1,4, diazoniabicyclo[2.2.2]octane-1,4-disulfinate; DABCO bis(sulfur dioxide) adduct; DABCO-bis(sulfur dioxide)
Selected publication
-
DABCO-Bis (Sulfur Dioxide), DABSO, as a Convenient Source of Sulfur Dioxide for Organic Synthesis: Utility in Sulfonamide and Sulfamide Preparation.
Woolven H.; González-Rodríguez C.; Marco I.; Thompson A.; Willis M. Org Lett 2011, 13 (18), 4876–4878. DOI: 10.1021/ol201957n
-
1,4-Disulfino-1,4-Diazabicyclo [2.2.2]Octane, Bis(Inner Salt).
Deeming A.; Willis M.; Lu T.; Xiang Y.; Wu J. Encyclopedia of Reagents for Organic Synthesis 2018, 1–15. DOI: 10.1002/047084289X.rn01912.pub2