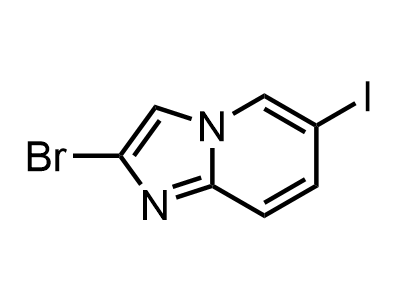
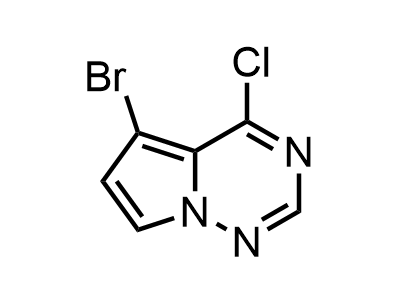
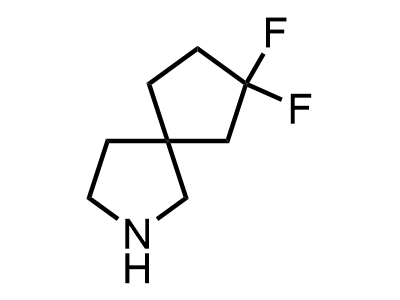
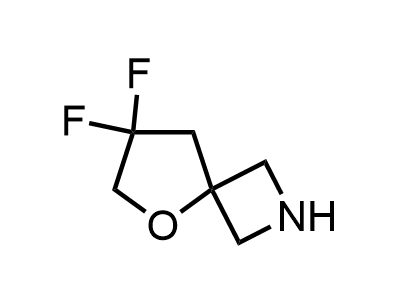
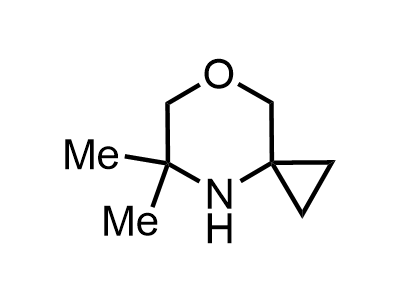
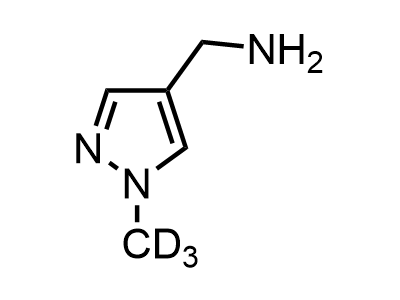
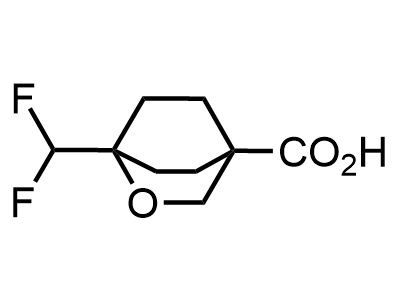
300 Thousand compounds in stock
Original and unique
Make-on-demand
Building Blocks
1B novel building blocks
Reliable supply
Over 650 highly skillful chemists
Unique synthesis technologies
48B Billion
REAL compounds and
Custom Library Synthesis
On site access to all Enamine stock BB’s
Highly flexible arrangements
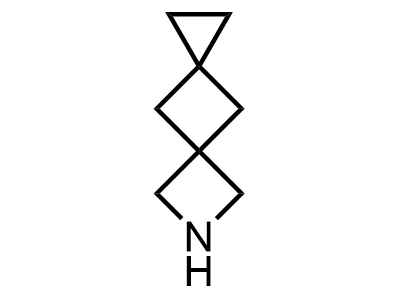
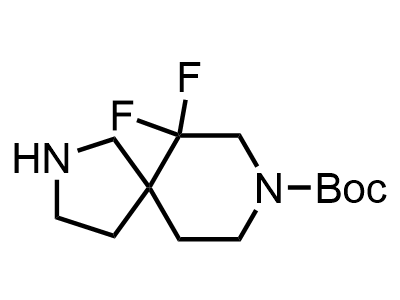
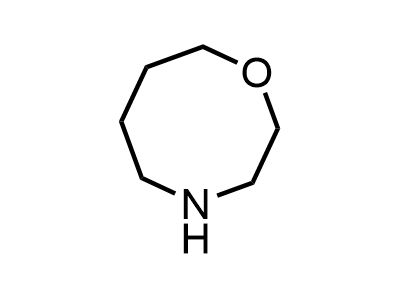
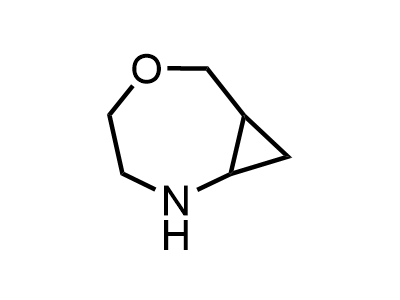
2 000 new building blocks are synthesized monthly. Here is an important update to our MedChem Highlights from March 2024
Recent News
11 April 2024
Press Release
Cambridge, UK and Kyiv, Ukraine, 11 April 2024: Metrion Biosciences Limited (“Metrion”), the specialist ion channel and cardiac safety screening contract research organisation (CRO) and drug discovery company, and Enamine Ltd (“Enamine”), the global leader in supplying small molecules and early drug discovery services, announced that Metrion has enhanced its High Throughput Screening (HTS) services with the addition of access to Enamine’s compound libraries.
27 March 2024
Press Release
March, 2024, Kyiv, Ukraine. Enamine Ltd, the global leader in supplying small molecules and early drug discovery services, announces the expansion of its library synthesis capabilities with a focus on Enamine REAL compounds to further support the growing demands of agricultural and pharmaceutical companies, research institutes, and drug discovery centers.
01 March 2024
News
We are excited to announce a strategic collaboration between Enamine, the world's leading provider of chemical building blocks, compound libraries, and biology services, and Genez International, a prominent enterprise with 15 years of experience in cross-border supply management, biopharmaceutical research and development, semiconductor equipment, and high-definition digital imaging systems.
Beilstein J. Org. Chem.
2017, 13, 2617-2625
DOI:
10.3762/bjoc.13.259
Melnykov S. V.; Pataman A. S.; Dmytriv Y. V.; Shishkina S. V.; Vovk M. V.; Sukach V. A.
Background: Due to the high reactivity towards various C-nucleophiles, trifluoromethylketimines are known to be useful reagents for the synthesis of α-trifluoromethylated amine derivatives. However, decarboxylative reactions with malonic acid and its mono(thio)esters have been poorly investigated so far despite the potential to become a convenient route to β-trifluoromethyl-β-amino acid derivatives and to their partially saturated heterocyclic analogues.
Results: In this paper we show that 4-trifluoromethylpyrimidin-2(1
H)-ones, unique heterocyclic ketimines, react with malonic acid under organic base catalysis to regioselectively provide either Michael- or Mannich-type decarboxylative addition products depending on solvent polarity. Malonic mono(thio)esters give exclusively Michael-type products. The two regioisomeric products can be converted into saturated (2-oxohexahydropyrimidin-4-yl)acetic acid derivatives by mild hydrogenation of the endocyclic C=C double bond in the presence of Pd/C as catalyst. The cis-stereoisomers selectively formed upon reduction of the Michael-type products were structurally determined by X-ray diffraction. As a result of this study, a number of novel acetic acid derivatives containing trifluoromethylated, partially or fully saturated 2-oxopyrimidine cores were prepared and characterized as promising building blocks.
Conclusions: Regio- and stereoselective protocols have been developed for the synthesis of novel isomeric 4(6)-trifluoromethylated 1,2,3,4-tetrahydro- and perhydro-(2-oxopyrimidin-4-yl)acetic acid derivatives.