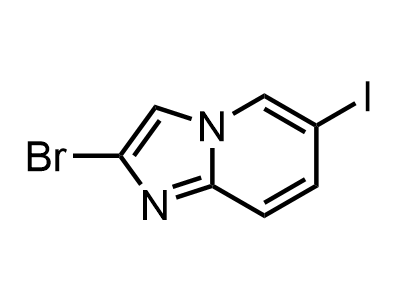
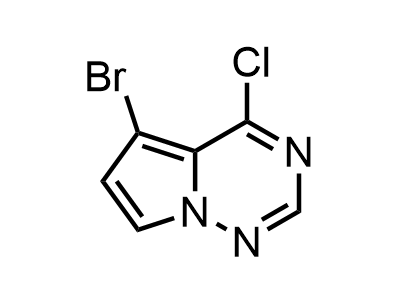
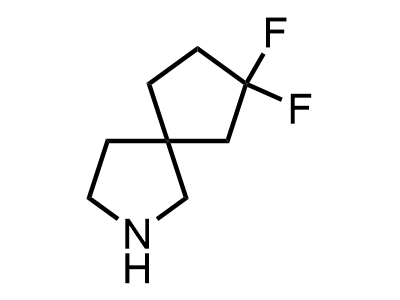
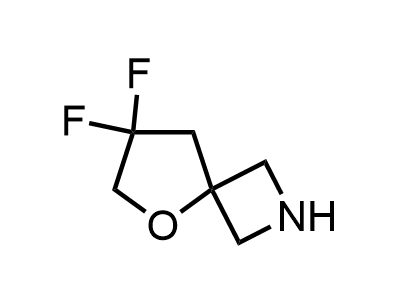
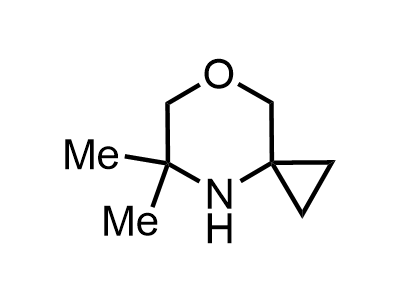
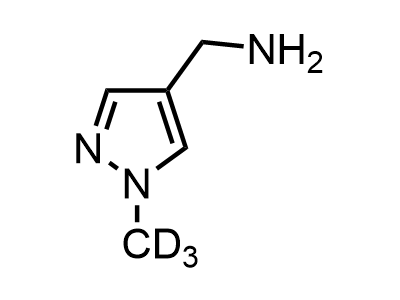
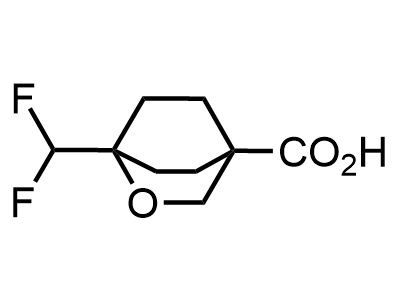
300 Thousand compounds in stock
Original and unique
Make-on-demand
Building Blocks
1B novel building blocks
Reliable supply
Over 650 highly skillful chemists
Unique synthesis technologies
48B Billion
REAL compounds and
Custom Library Synthesis
On site access to all Enamine stock BB’s
Highly flexible arrangements
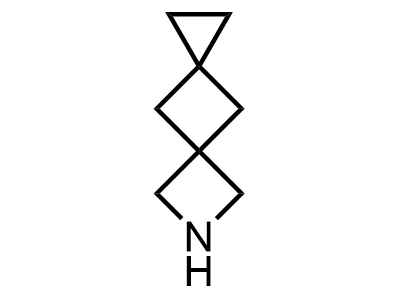
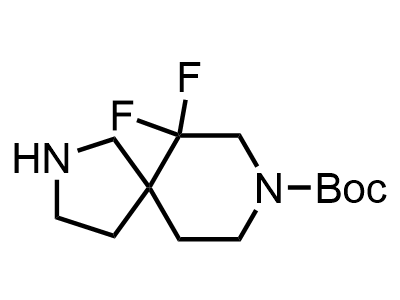
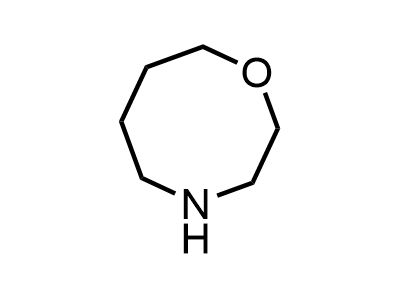
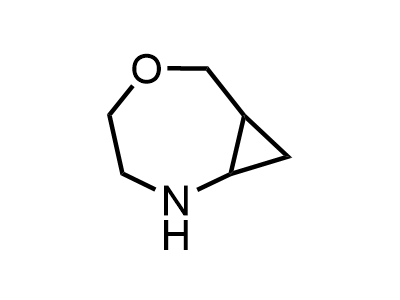
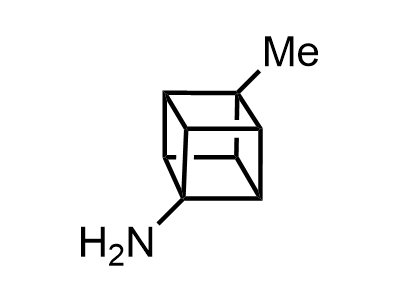
2 000 new building blocks are synthesized monthly. Here is an important update to our MedChem Highlights from March 2024
Recent News
11 April 2024
Press Release
Cambridge, UK and Kyiv, Ukraine, 11 April 2024: Metrion Biosciences Limited (“Metrion”), the specialist ion channel and cardiac safety screening contract research organisation (CRO) and drug discovery company, and Enamine Ltd (“Enamine”), the global leader in supplying small molecules and early drug discovery services, announced that Metrion has enhanced its High Throughput Screening (HTS) services with the addition of access to Enamine’s compound libraries.
27 March 2024
Press Release
March, 2024, Kyiv, Ukraine. Enamine Ltd, the global leader in supplying small molecules and early drug discovery services, announces the expansion of its library synthesis capabilities with a focus on Enamine REAL compounds to further support the growing demands of agricultural and pharmaceutical companies, research institutes, and drug discovery centers.
01 March 2024
News
We are excited to announce a strategic collaboration between Enamine, the world's leading provider of chemical building blocks, compound libraries, and biology services, and Genez International, a prominent enterprise with 15 years of experience in cross-border supply management, biopharmaceutical research and development, semiconductor equipment, and high-definition digital imaging systems.
Angew. Chem. Int. Ed.
2016, 55 (47), 1459514599
DOI:
10.1002/anie.201607161
Michurin O. M.; Afonin S.; Berditsch M.; Daniliuc C. G.; Ulrich A. S.; Komarov I. V.; Radchenko D. S.
Conformationally constrained non-racemizing trifluoromethyl-substituted lysine isosteres [(E)- and (Z)-TCBLys] with charged side chains are presented as a new type of 19F-NMR labels for peptide studies. Design of the labels, their synthesis, incorporation into peptides and experimental demonstration of their application for solid state NMR studies of membrane-active peptides are described. A series of fluorine-labeled analogues of the helical amphipathic antimicrobial peptide PGLa(Nle) was obtained, in which different lysine residues in the original peptide sequence were replaced, one at a time, by either (E)- or (Z)-TCBLys. Antimicrobial activities of the synthesized analogues were practically the same as those of the parent peptide. The structural and orientational parameters of the helical PGLa(Nle) peptide in model bilayers, as determined using the novel labels confirmed and refined the previously known structure. (E)- and (Z)-TCBLys, as a set of cationic 19F-NMR labels, were shown to deliver structural information about the charged face of amphipathic peptides by solid state 19F-NMR, previously inaccessible by this method.