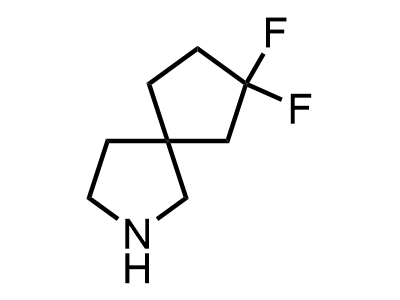
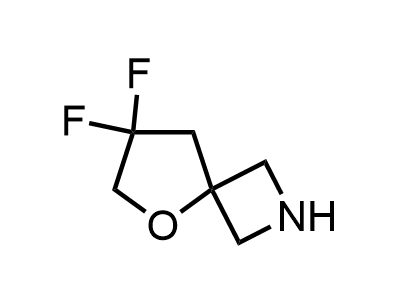
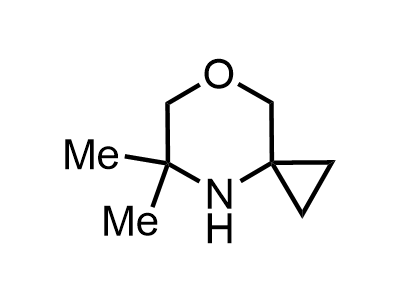
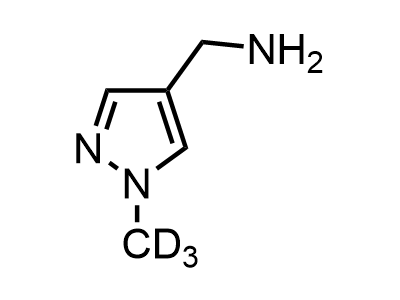
300 Thousand compounds in stock
Original and unique
Make-on-demand
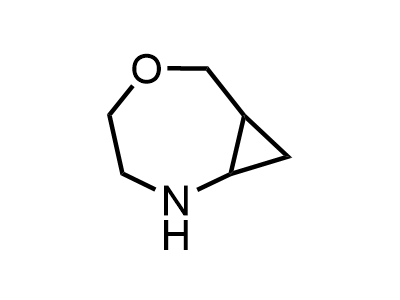
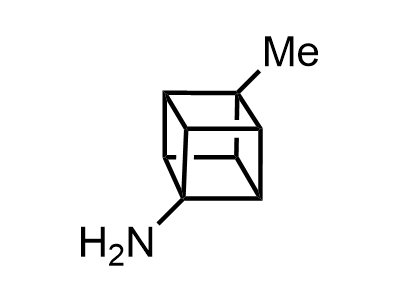
Building Blocks
1B novel building blocks
Reliable supply
Over 650 highly skillful chemists
Unique synthesis technologies
48B Billion
REAL compounds and
Custom Library Synthesis
On site access to all Enamine stock BB’s
Highly flexible arrangements
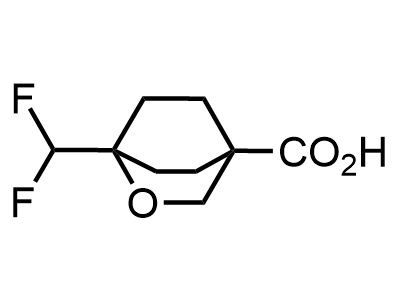
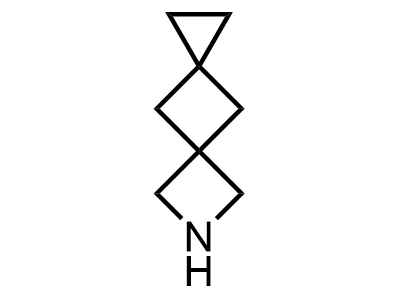
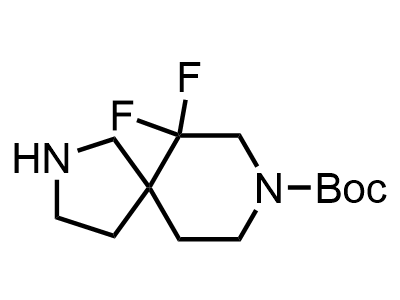
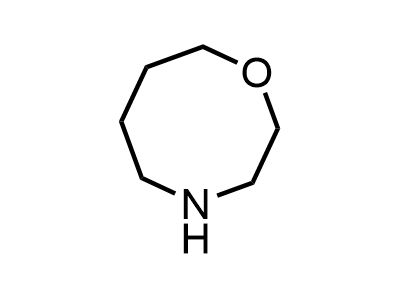
2 000 new building blocks are synthesized monthly. Here is an important update to our MedChem Highlights from March 2024
Recent News
11 April 2024
Press Release
Cambridge, UK and Kyiv, Ukraine, 11 April 2024: Metrion Biosciences Limited (“Metrion”), the specialist ion channel and cardiac safety screening contract research organisation (CRO) and drug discovery company, and Enamine Ltd (“Enamine”), the global leader in supplying small molecules and early drug discovery services, announced that Metrion has enhanced its High Throughput Screening (HTS) services with the addition of access to Enamine’s compound libraries.
27 March 2024
Press Release
March, 2024, Kyiv, Ukraine. Enamine Ltd, the global leader in supplying small molecules and early drug discovery services, announces the expansion of its library synthesis capabilities with a focus on Enamine REAL compounds to further support the growing demands of agricultural and pharmaceutical companies, research institutes, and drug discovery centers.
01 March 2024
News
We are excited to announce a strategic collaboration between Enamine, the world's leading provider of chemical building blocks, compound libraries, and biology services, and Genez International, a prominent enterprise with 15 years of experience in cross-border supply management, biopharmaceutical research and development, semiconductor equipment, and high-definition digital imaging systems.
J. Org. Chem.
2011, 76 (14), 5774-5781
DOI:
10.1021/jo2008252
Ryabukhin S.; Naumchik V.; Plaskon A.; Grygorenko O.; Tolmachev A.
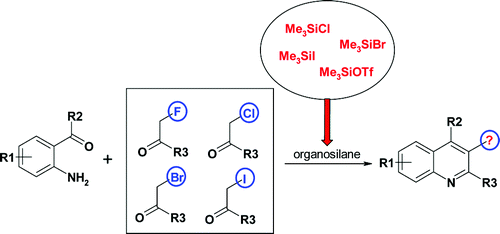
A general approach to 3-fluoro-, 3-chloro-, and 3-bromoquinolines which relies on organosilane-promoted Friedlander reaction of alpha-haloketones is described. The scope of the methylene component as well as influence of the organosilane component on the outcome of the reaction is studied. The method can be used under parallel synthesis conditions.